Kidney Damage
Can I Get Social Security Disability Benefits for Kidney Damage?
- About Kidney Damage and Disability
- How to Get Disability Benefits for Kidney Damage by Meeting a Listing
- Residual Functional Capacity Assessment for Kidney Damage
- Getting Your Doctor’s Medical Opinion About What You Can Still Do
If you have kidney damage, Social Security disability benefits may be available. To determine whether you are disabled by your kidney damage, the Social Security Administration first considers whether it is severe enough to meet or equal a listing at Step 3 of the Sequential Evaluation Process. See How to Get Disability Benefits for Kidney Damage by Meeting a Listing. If you meet or equal a listing because of your kidney damage, you are considered disabled. If your kidney damage is not severe enough to equal or meet a listing, the Social Security Administration must assess your residual functional capacity (RFC) (the work you can still do, despite the kidney damage), to determine whether you qualify for disability benefits at Step 4 and Step 5 of the Sequential Evaluation Process.
About Kidney Damage and Disability
To satisfy the SSA’s listing for kidney damage, the kidneys must be permanently damaged; there must be chronic renal failure (CRF). Acute renal failure (ARF) cannot qualify, since it cannot fulfill duration. There are many possible causes of ARF. Most are associated with trauma, surgery, and acute medical illnesses. If reversibility is not attained, the ARF becomes CRF. However, many cases of CRF are of gradual progression to end-stage renal disease (ESRD). ESRD refers to a condition in which kidney function is so minimal that it is non-existent for all practical purposes. In the U.S., there are roughly half a million people with ESRD.
A person can have substantial kidney damage before it begins to cause problems. Symptoms will not appear until the great majority of kidney cells are destroyed. For example, a person can survive normally with only one kidney and no symptoms. That is why is possible to donate a kidney. See Residual Functional Capacity Assessment for Kidney Damage.
Basics of Kidney Function
The urinary tract consists of the kidneys, ureters, bladder, and urethra. Urine is an ultrafiltrate of blood concentrated by the kidneys as waste material. A ureter drains the urine from each kidney and carries it to the bladder where it is stored. The urethra allows the bladder to be drained to the outside of the body.
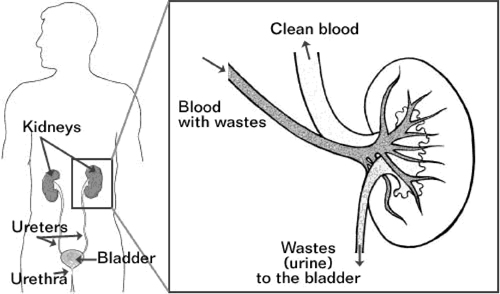
Genito-Urinary Tract, Gross Anatomy
Each of the normal body’s two kidneys contains about one million filtering units called nephrons; this is a highly redundant system, since one kidney is sufficient for the body’s needs. A large artery (renal artery) connects each kidney to the aorta, and supplies the kidneys with a high blood flow comprising 25% of the entire cardiac output of blood. The kidneys not only filter toxic metabolic products from the bloodstream, but they also play important roles in maintaining the body’s acidity, fluid and electrolyte (e.g., potassium, sodium, chloride) balance, and also have significant hormonal functions. Renal glomeruli are microscopic ball-shaped tufts of blood vessels. Each glomerulus is surrounded by a cup-shaped structure known as Bowman’s capsule. Bowman’s capsule is the expanded end of a kidney tubule. Water and waste substances can exit the bloodstream from the glomerulus and enter Bowman’s capsule. A nephron consists of one glomerulus along with Bowman’s capsule, associated tubule, and collecting duct for the final product of filtration (urine).
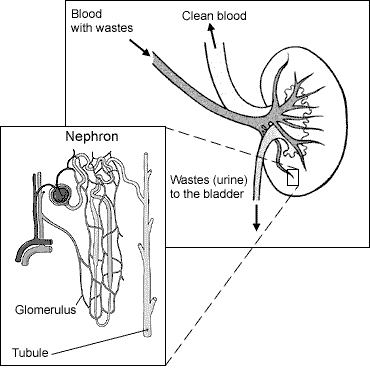
Genito-Urinary Tract, MicroAnatomy of Nephron
The glomerular filtration rate (GFR) is an important measure of kidney function, because it measures how effectively blood is being filtered by the kidney. The tubule connected to Bowman’s capsule makes a curve called the loop of Henle. The loop of Henle is involved in a complex exchange process of water, sodium chloride, urea, and other substances with the surrounding interstitium of the kidney. The loop of Henle ends as a collecting duct for the final urine produced by each nephron. Then, the million collecting ducts from the various nephrons eventually drain into common areas of the kidney known as calyces that in turn form the renal pelvis that drains into the ureter and the bladder.
Abnormalities Associated with Chronic Renal Failure
The toxic state caused by advanced renal failure is called uremia (azotemia). In chronic renal failure, numerous abnormalities in various degrees of severity are possible, including:
- fatigue
- loss of appetite (anorexia)
- weight loss
- generalized itching (pruritis)
- general feeling of sickness (malaise)
- lethargy
- sleepiness (somnolence)
- decreased alertness
- pericarditis
- increased skin pigmentation (especially yellow or brown)
- easy bruisability
- bone disease (renal osteodystrophy)
- frequent hiccups
- increased or decreased urine output6
- muscle cramps or twitching (peripheral motor neuropathy)
- increased nighttime urination (nocturia)
- decreased touch sensation (peripheral sensory neuropathy)
- seizures
- headache
- confusion
- hypertension
- bleeding
- anemia
- fluid retention (edema)
- coma
- death
Chronic Nephritis
Nephritis is a non-specific term referring to the various forms of disease affecting the kidney’s glomeruli, more commonly known as glomerulonephritis (GN). Depending on the individual and specific form of GN, the disease can be acute and reversible or rapidly progress into kidney failure. However, “nephritis” may also refer to inflammatory lesions elsewhere in the kidney other than glomeruli—interstitial nephritis.
Polycystic Disease
In most cases of polycystic kidney disease, there is inheritance from one parent (autosomal dominant heredity) based on gene abnormalities on chromosomes 4 and 16, and possibly others. This disorder involves parts of the kidney being replaced with non-functional cysts or cavities that may be filled with fluid. Polycystic disease is not rare; the autosomal dominant form (ADPKD) occurs in about 500 to 1000 individuals and is a kidney disorder frequently seen by the SSA. This is often a slowly progressive impairment, with end-stage renal disease (ESRD) sometimes not occurring until old age (70+ years). Others are less fortunate, although the anemia associated with other types of renal failure is often not as severe in polycystic disease. In fact, the abnormal cystic tissue may actually produce above normal amounts of erythropoietin to stimulate bone marrow red cell production.
The diagnosis of polycystic disease is not difficult and claimants with this disorder have almost always been diagnosed by the treating physicians before they apply for disability. Large and irregular shaped kidney cysts may be palpable on physical examination; others can be diagnosed by CT scans, abdominal ultrasound (the SSA can easily purchase ultrasound testing if needed), magnetic resonance scans, or intravenous urography. However, benign kidney cysts having nothing to do with polycystic kidney disease are extremely common with increasing age, although rare in individuals under age 30. In this group, there should be at least two cysts in one kidney for the diagnosis of polycystic kidney disease to be made. In the 30–59 age range, there should be at least two cysts in each kidney. In those individuals 60 years or more of age, there should be at least four cysts in each kidney for the diagnosis of polycystic disease to be made.
Diabetes Mellitus
Diabetes is by far the most frequent cause of chronic renal failure and ESRD, principally through its damage to glomeruli. This is an extremely unfortunate consequence of diabetes, because the progression of diabetic kidney disease is generally related to how well the diabetes is controlled. Since so many diabetics are under poor control, either because of non-compliance with prescribed therapy or an interfering impairment such as a mental disorder, the SSA sees many cases of renal failure caused by diabetes. In some people, diabetic kidney disease is relentlessly progressive, even though blood sugars are closely controlled with insulin or other medication. Kidney failure can occur in both insulin-dependent (IDDM) and non-insulin dependent (NIDDM) diabetes mellitus. About 40% of people with diabetic kidney disease have ESRD by the time they are 50 years of age.
Five Stages of Diabetic Kidney Disease
Scientists have described five stages in the progression to ESRD in people with diabetes. They are as follows:
- Stage I. The flow of blood through the kidneys, and therefore through the glomeruli, increases—this is called hyperfiltration—and the kidneys are larger than normal. Some people remain in stage I indefinitely; others advance to stage II after many years.
- Stage II. The rate of filtration remains elevated or at near-normal levels, and the glomeruli begin to show damage. Small amounts of a blood protein known as albumin leak into the urine—a condition known as microalbuminuria. In its earliest stages, microalbuminuria may come and go. But as the rate of albumin loss increases from 20 to 200 micrograms per minute, microalbuminuria becomes more constant. (Normal losses of albumin are less than 5 micrograms per minute.) A special test is required to detect microalbuminuria. People with NIDDM and IDDM may remain in stage II for many years, especially if they have normal blood pressure and good control of their blood sugar levels.
- Stage III. The loss of albumin and other proteins in the urine exceeds 200 micrograms per minute. It now can be detected during routine urine tests. Because such tests often involve dipping indicator strips into the urine, they are referred to as “dipstick methods.” Stage III sometimes is referred to as “dipstick-positive proteinuria” (or “clinical albuminuria” or “overt diabetic nephropathy”). Some patients develop high blood pressure. The glomeruli suffer increased damage. The kidneys progressively lose the ability to filter waste, and blood levels of creatinine and urea-nitrogen rise. People with IDDM and NIDDM may remain at stage III for many years.
- Stage IV. This stage is referred to as “advanced clinical nephropathy.” The glomerular filtration rate decreases to less than 75 milliliters per minute, large amounts of protein pass into the urine, and high blood pressure almost always occurs. Levels of creatinine and urea-nitrogen in the blood rise further.
- Stage V. The final stage of diabetic neuropathy is ESRD. The glomerular filtration rate drops to less than 10 milliliters per minute. Symptoms of kidney failure occur.
These stages describe the progression of kidney disease for most people with IDDM who develop ESRD. For people with IDDM, the average length of time required to progress from onset of kidney disease to stage IV is 17 years. The average length of time to progress to ESRD is 23 years. Progression to ESRD may occur more rapidly (5–10 years) in people with untreated high blood pressure. If proteinuria does not develop within 25 years, the risk of developing advanced kidney disease begins to decrease. Advancement to stages IV and V occurs less frequently in people with NIDDM than in people with IDDM. Nevertheless, about 60 percent of people with diabetes who develop ESRD have NIDDM.
Dialysis
When the kidneys fail to function in ESRD, they can no longer filter waste products from the blood. Left untreated, ESRD will result in such an accumulation of toxic metabolic products that an individual will become quite ill within a few days and then enter a coma with resultant death. If ESRD is present, there is no option but to have dialysis or a kidney transplant. Since there are not enough donor kidneys available for needy recipients with ESRD, many patients receive dialysis. Dialysis removes toxic substances from the blood. ESRD is reached in men when the serum creatinine is about 10–12 mg/dl and in women about 7–8 mg/dl, and planning for dialysis or transplantation should start when the serum creatinine reaches 4 to 6 mg/dL in women and 6 to 8 mg/dL in men. Normal serum creatinine levels in both sexes is less than 1.5 mg/dl.
There are two general types of dialysis. One is hemodialysis, in which the blood is filtered directly. A permanent connection (fistula) is surgically made between the radial artery and a vein in the wrist. This arteriovenous (AV) shunt allows higher pressure arterial blood to flow into the vein, which then serves as a connection point for hemodialysis. Shunts are placed in anticipation of eventual dialysis when the serum creatinine rises above 4 mg/dl or creatinine clearance drops below 15–20 ml/min. By insertion of a needle into the AV shunt, blood can be removed and filtered of waste materials at regular intervals by passage through membranes in a machine and then returning it to the body—a process that takes about 2–4 hours. Most patients require hemodialysis 3 times a week.
The Basics of Hemodialysis: Figure 6.02.1.a – 11 (Medical Issues in Social Security Disability by Morton)
In peritoneal dialysis, special fluids (dialysate) are put into the abdominal cavity. There is a smooth membrane which lines the abdominal cavity, called the peritoneum. Waste materials from the blood will cross the peritoneum naturally and be picked up by the fluids, which are then removed and discarded.
Ambulatory Peritoneal Dialysis: Figure 6.02.1.a – 12 (Medical Issues in Social Security Disability by Morton)
In chronic ambulatory peritoneal dialysis (CAPD), patients can perform peritoneal dialysis on themselves and go about their business after placing the dialysis fluid into their own abdominal cavity through an indwelling port that has been surgically placed. The dialysate stays in the abdomen about 4–6 hours, and is exchanged about 4 times daily. It takes 30–40 minutes to drain the used dialysate and replace it with a fresh solution.
In chronic cyclic peritoneal dialysis (CCPD), the dialysate exchanges are done automatically by a machine while the person sleeps, and takes about 10–12 hours every night. Intermittent peritoneal dialysis (IPD) is similar to CCPD, except that it is usually performed in a hospital.
ESRD is dangerous even when dialysis is received.
Amyloidosis
Proteins are important building blocks for all body parts, including muscles, bones, hair, and nails. Proteins circulate throughout the body in the blood and are normally harmless. Occasionally, cells produce abnormal proteins that can settle in body tissue, forming deposits and causing disease. When these deposits of abnormal proteins were first discovered, they were called amyloid, and the disease process amyloidosis.
In recent years, researchers have discovered that different kinds of proteins can form amyloid deposits and have identified several types of amyloidosis. Two of these types are closely related to kidney disease. In primary amyloidosis, abnormal protein production occurs as a first step and can lead to kidney disease. Dialysis-related amyloidosis (DRA), on the other hand, is a result of kidney disease.
Primary Amyloidosis
Primary amyloidosis occurs when the body produces abnormal protein fibers, which join together to form amyloid deposits in different organs, including the kidneys, where they cause serious damage. Injured kidneys cannot function effectively and may be unable to remove urea and other wastes from the blood. Elevated levels of these waste products can also damage the heart, lungs, brain, and digestive system.
One common sign of amyloidosis is the presence of abnormally high amounts of protein in the urine, a condition known as proteinuria. Healthy kidneys prevent protein from entering the urine, so the presence of protein may be a sign that the kidneys aren’t working properly. A physician who finds large amounts of protein in the urine may also perform a biopsy—take a small sample of tissue for examination under a microscope—to confirm amyloidosis.
No effective treatment has been found to reverse the effects of amyloidosis. Combination drug therapy with melphalan (a cancer drug) and prednisone (an anti-inflammatory steroid drug) may improve organ function and survival rates by interrupting the growth of cells that produce amyloid protein. These are the same drugs used in chemotherapy to treat certain cancers, and they may have serious side effects, such as nausea and vomiting, hair loss, and fatigue.
Dialysis-Related Amyloidosis
Normal kidneys filter excess proteins from the blood, thus preventing levels from getting too high. When the kidneys don’t work properly, as in patients receiving dialysis, another type of protein called beta-2-microglobulin may build up in the blood. When this occurs, beta-2-microglobulin molecules may join together, like the links of a chain, forming a few very large molecules from many smaller ones. These large molecules can form deposits and eventually damage the surrounding tissues and cause great discomfort. This condition is called dialysis-related amyloidosis (DRA).
DRA is relatively common in patients who have been on dialysis for more than 5 years, especially among the elderly. Dialysis membranes don’t effectively remove the large, complex beta-2-microglobulin proteins from the bloodstream. As a result, blood levels become elevated, and deposits form in bone, joints, and tendons. DRA may result in pain, stiffness, and fluid in the joints. Patients with DRA may also develop hollow cavities, or cysts, in some of their bones; these may lead to unexpected bone fractures. Amyloid deposits may cause tears in ligaments and tendons (the tissue that connects the muscle to the bone). Most patients with these problems can be helped by surgical intervention.
Half of the people with DRA also develop a condition called carpal tunnel syndrome, which results from the unusual buildup of protein in the wrists. Patients with this disorder may experience numbness or tingling, sometimes associated with muscle weakness, in their fingers and hands. This is a treatable condition. Amyloid may build up in the wrist and cause bone cysts or carpal tunnel syndrome. Unfortunately, no cure for DRA has been found, although a successful kidney transplant may stop the disease from progressing. However, DRA has caught the attention of dialysis engineers who are attempting to develop membranes that can more efficiently remove beta-2-microglobulin from the blood.
Continue to How to Get Disability Benefits for Kidney Damage by Meeting a Listing.
